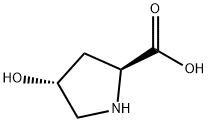
L-Hydroxyproline CAS# 51-35-4
Hydroxyproline is a non-essential amino acid divided from another amino acid called proline. It is created by the communication of proline with ascorbic acid vitamin C. This process produces a hydroxyl team bound to a hydrogen-oxygen particle, which is affixed to a carbon particle of proline as well as converted to hydroxyproline.Trans-4-hydroxy-l-proline is an optically energetic form of 4-hydroxyproline with l-trans setup. It can be used as human metabolite, plant metabolite and also computer mouse metabolite. It is an tautomer of trans-4-hydroxy-l-proline zwitterions.
发送询盘
L-Hydroxyproline CAS# 51-35-4
| Product Name: | L-Hydroxyproline |
| Synonyms: | (S)-(-)-TRANS-4-HYDROXYPROLINE FOR SYNTH;trans-4-Hydroxy-L-proline, 99+% 10GR;trans-4-Hydroxy-L-proline, 99+% 25GR;trans-4-Hydroxy-L-proline Microbial, cell culture tested;4-HYDROXY-2-PYRROLIDINECARBOXYLIC ACID;4-HYDROXY-L-PROLINE TRANS ISOMER;(S)-(-)-TRANS-4-HYDROXYPROLINE;RARECHEM EM WB 0135 |
| CAS: | 51-35-4 |
| MF: | C5H9NO3 |
| MW: | 131.13 |
| EINECS: | 200-091-9 |
| Product Categories: | Amino Acids;Pyrrole&Pyrrolidine&Pyrroline;Hydroxyproline [Hyp];Unusual Amino Acids;Biochemistry;Biological-modified Amino Acids;L-Amino Acids;PHARMACEUTICALS;Nitrogen cyclic compounds;Amino Acids;Amino Acids & Derivatives;Chiral Reagents;Heterocycles;bc0001;51-35-4 |
| Mol File: | 51-35-4.mol |
 |
|
| L-Hydroxyproline Chemical Properties |
| Melting point | 273???C (dec.)(lit.) |
| alpha | -75.5 o (c=5, H2O) |
| Boiling point | 242.42??C (rough estimate) |
| density | 1.3121 (rough estimate) |
| vapor density | 4.5 (vs air) |
| refractive index | -75.5 ?? (C=4, H2O) |
| storage temp. | Store below +30??C. |
| solubility | H2O: 50?mg/mL |
| form | Crystals or Crystalline Powder |
| pka | 1.82, 9.66(at 25??) |
| color | White |
| PH | 5.5-6.5 (50g/l, H2O, 20??) |
| Odor | Odorless |
| optical activity | [??]25/D 75.6??, c = 1 in H2O |
| Water Solubility | 357.8 g/L (20 o C) |
| Merck | 14,4840 |
| BRN | 471933 |
| InChIKey | PMMYEEVYMWASQN-DMTCNVIQSA-N |
| LogP | -0.350 (est) |
| CAS DataBase Reference | 51-35-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydroxyproline(51-35-4) |
| EPA Substance Registry System | trans-4-Hydroxy-L-proline (51-35-4) |
- 2
- 2-diallylpent-4-en-1-amine
- 4
- 95-16-9
- Ammonium sulfamate
- Benzothiazole
- cas:67889-00-3ح2
- cas:83524-75-8 | pigment black 32
- cas:928836-00-4 | 2
- cas:932745-70-5 | 4
- Chemical Minerals
- Coconut diethanolamide
- Daily Chemicals
- discount
- for sale
- General pvc resin
- hexyl D-glucoside
- in stock
- Lauramidopropyl betaine
- LAURIC ACID MONOETHANOLAMIDE
- Petroleum Additives
- Plasticiser
- Ploymers
- price
- PVC
- quotation
- Raw Materal
- Remove term: Petroleum Additives Petroleum Additive
- SODIUM ETHYL 2-SULFOLAURATE
Related Products
Chemical Name: Imazalil Sulfate
CAS No.: 58594-72-2
Molecular Formula: C14H14Cl2N2O.H2SO4
Molecular Weight: 395.26
Appearance: Solid
Chemical Name: Arabic gum
CAS No.: 9000-01-5
Appearance: powder
Chemical Name: Zinc citrate
Synonyms: Zinc citrate trihydrate
CAS No.: 546-46-3
Molecular Formula: C6H8O7Zn
Molecular Weight: 257.5
Appearance: White powder
Polyglutamic acid (y-PGA), also known as natto gum, is a high molecular peptide polymer synthesized from several glutamic acid monomers through microbial fermentation. It is rich in glutamic acid, glucose, protein and minerals. , vitamins and other biologically active substances.
Polyglutamic acid (??-PGA) is a sticky substance that was first discovered in ??Natto??. It is currently widely used in agricultural production and is called a new biostimulant. It is fully water-soluble, biodegradable, edible, and non-toxic. It is a biopolymer produced by microbial fermentation.
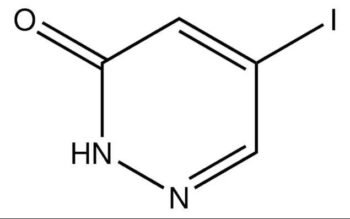
Common English name: 5-iodo-2,3-dihydropyridazin-3-one
CAS No.: 825633-94-1
Molecular formula: C4H3IN2O
Molecular weight: 221.98
Sample: Available
EC 3.4.21.14, previously classified, now redirects to EC 3.4.21.67, identifying endopeptidase So. This serine endopeptidase is integral in the hydrolysis of peptide bonds, a critical function in biological systems. Its applications extend across research and development in the pharmaceutical and biotechnological industries.
Chemical Name: Dehydrocholic acid
Synonyms: Acide dehydrocholique; Triketocholanic acid
CAS No.: 81-23-2
Molecular Formula: C24H34O5
Molecular Weight: 402.53
Appearance: Powder
Chemical Name: 3-Hydroxybutyric acid
CAS No.: 625-71-8
Molecular Formula: C4H8O3
Molecular Weight: 104.1
Appearance: White powder
Coenzyme A sodium salt hydrate (CAS 55672-92-9) is an important biologically active substance.
Appearance: Usually white or off-white powder. Solubility: Easily soluble in water, forming a clear solution in water.
Function: In the body, coenzyme A sodium salt hydrate is an important coenzyme that participates in a variety of biochemical reactions. It plays a key role in the metabolism of fatty acids, promoting the activation and oxidative decomposition of fatty acids. It participates in the tricarboxylic acid cycle and provides energy for cells. It is also important for the metabolism of certain amino acids.
Application: Commonly used in biochemistry and molecular biology research as a cofactor for enzyme reactions.
In the field of medicine, it may be used in the treatment or adjuvant treatment of certain diseases.
Product name:Cyclopentane
Purity:96%
Appearance:White powder
Package:25kg/bag
Sample:Available
Copper acetate peptide, also known as blue copper peptide. Copper peptide, also known as tripeptide in Chinese; Glycyl-L-histomyl-L-lysine. Peptide is a small molecule protein composed of amino acids, which are more easily absorbed by the skin and have more significant effects. It was first isolated from human plasma in 1973 and was discovered to have wound repair function in 1985. In 1999, researchers believed that copper peptide and its copper repair products can serve as activators of tissue remodeling, and it is also a signaling peptide, Promote the degradation of a large amount of collagen aggregates outside scars, the synthesis of normal collagen in the skin, the generation of elastin, proteoglycans, and glucosamine glycans, the growth rate and migration of different cell types, anti-inflammatory, and antioxidant responses.
EC 3.4.21.14, previously classified, now redirects to EC 3.4.21.67, identifying endopeptidase So. This serine endopeptidase is integral in the hydrolysis of peptide bonds, a critical function in biological systems. Its applications extend across research and development in the pharmaceutical and biotechnological industries.